Search history
Clear allSearch by image
XDrag and drop an image here or upload an image
Max 5MB per image
UploadSign In | Join
Search history
Clear allSearch by image
XDrag and drop an image here or upload an image
Max 5MB per image
UploadSign In | Join
X Email Mobile
 kx1103-a fruit battery a (blister)
kx1103-a fruit battery a (blister)
|
CN¥ 5.9 |
 kx1103-b fruit battery b (blister)
kx1103-b fruit battery b (blister)
|
CN¥ 8.9 |
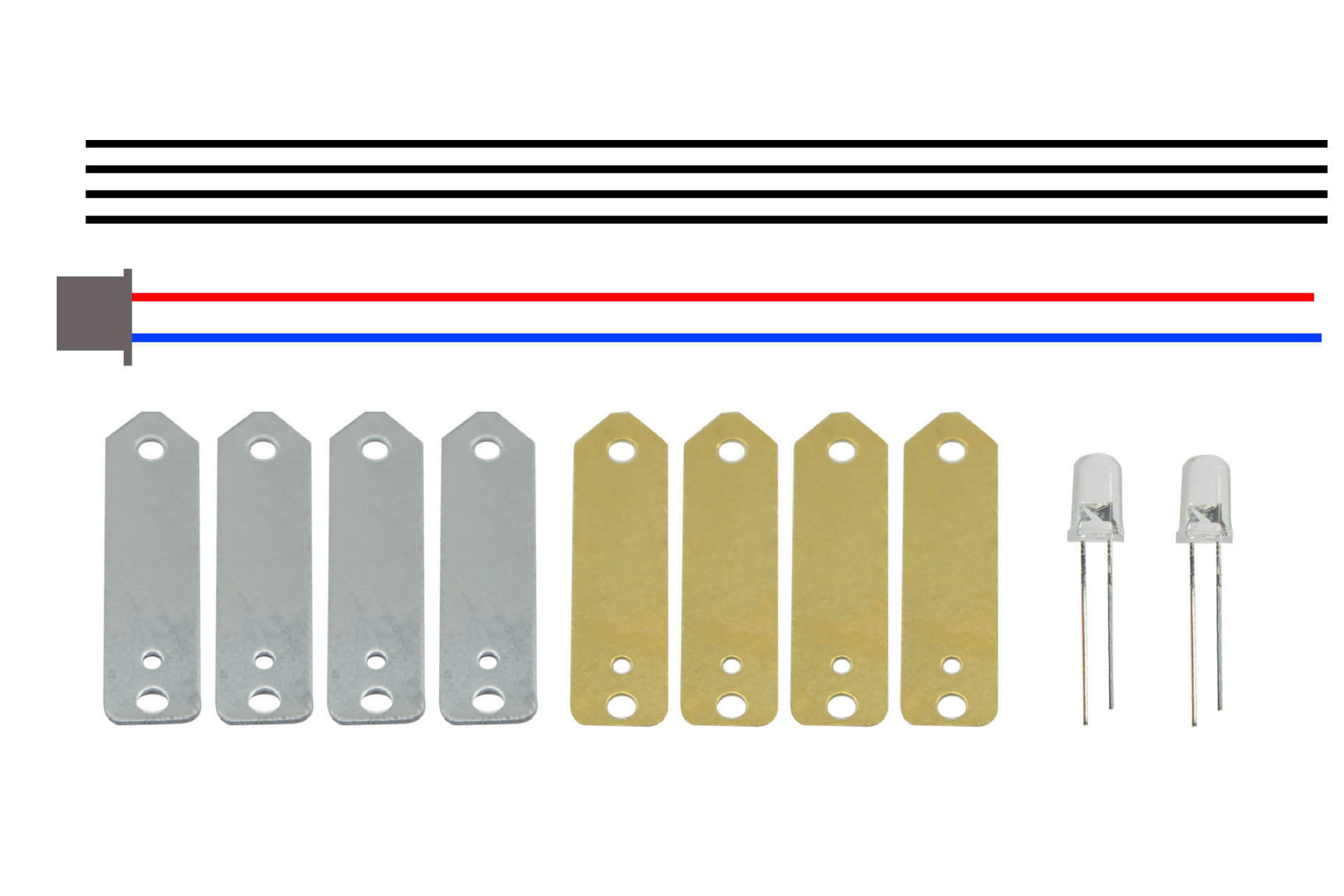
Product Code: KX1103-A
Product Name: Fruit Power A (Squeeze)
Size: Approx. 140*95*24mm
Weight: About 7KG
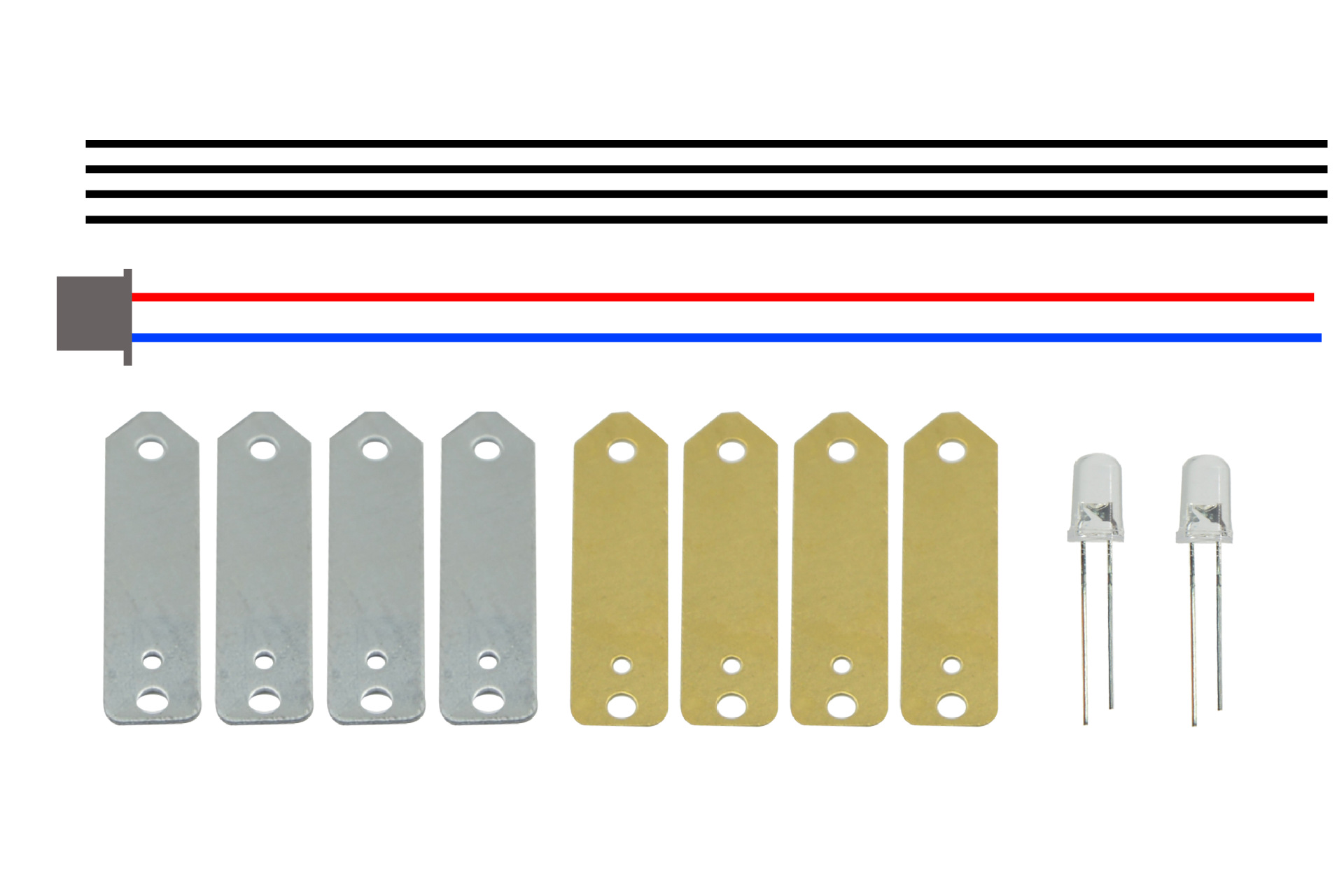
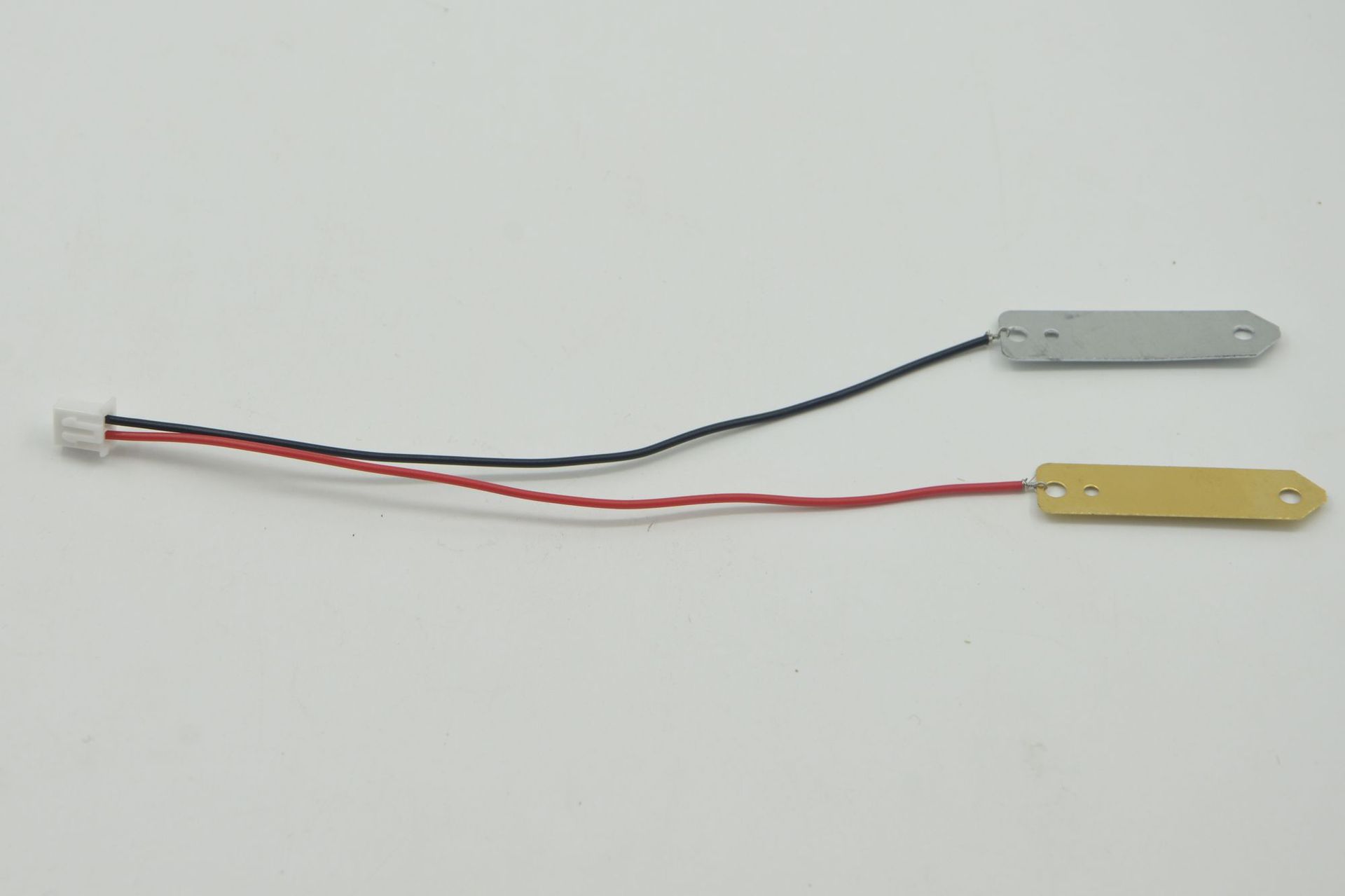
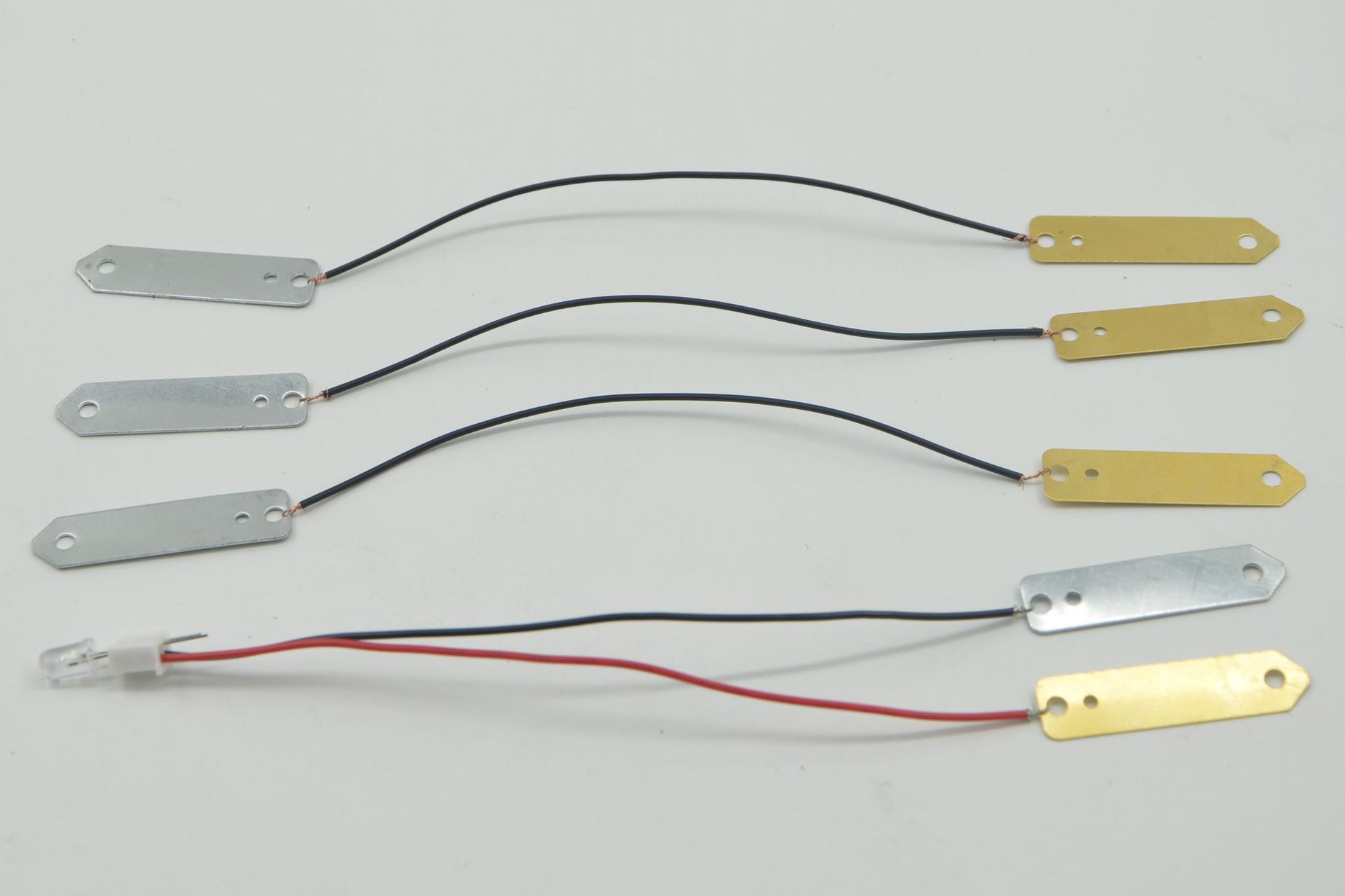
Material: copper sheets, zinc sheets, terminals, wires, LEDs
Color: Mixed
Battery Required: No
Packing: 240 pcs
Box Specification: 360*360*415 mm

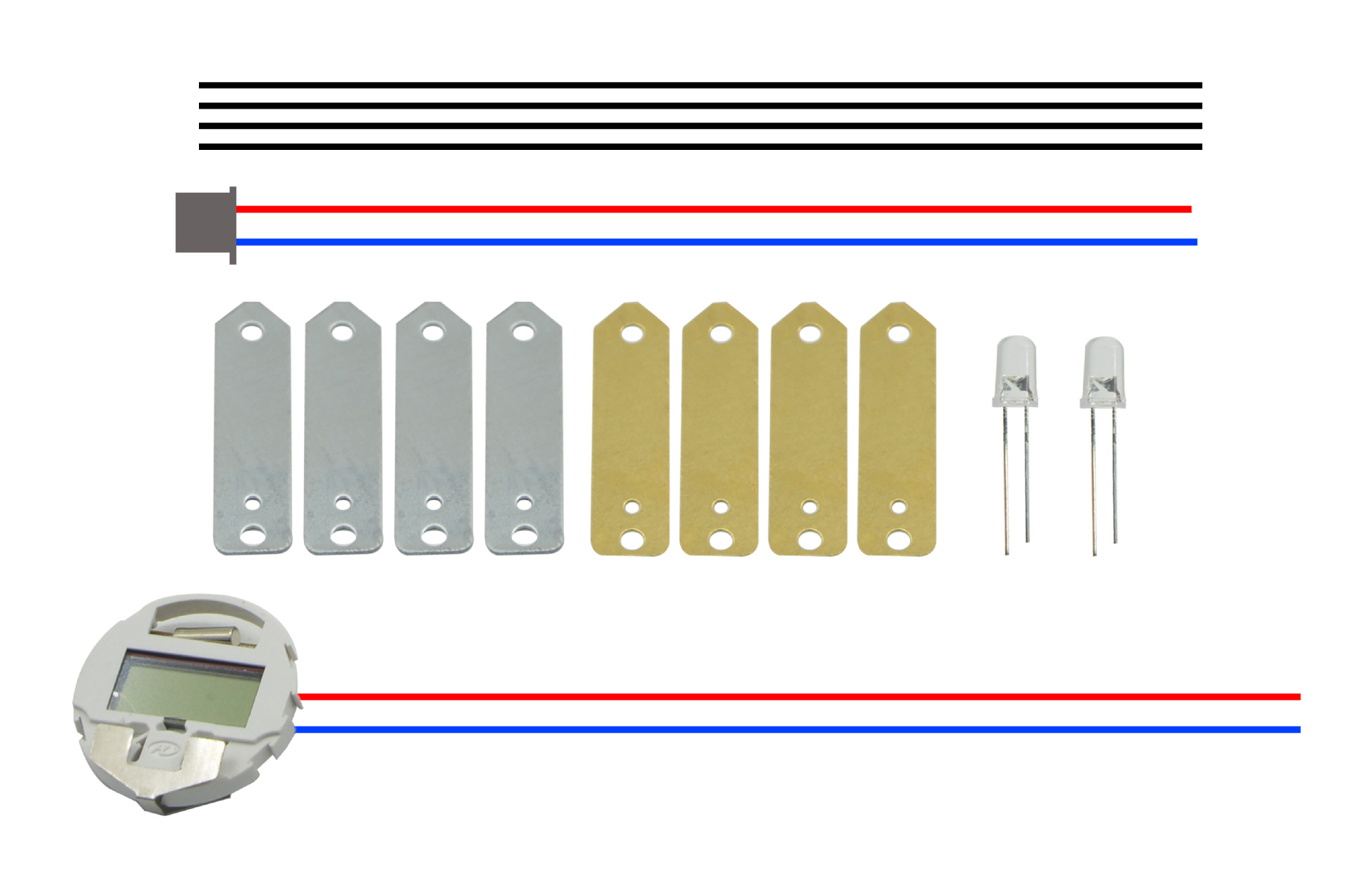
Product Code: KX1103-B
Product Name: Fruit Power B (Squeeze)
Size: Approx. 140*95*24mm
Weight: About 8KG
Material: copper sheets, zinc sheets, terminal wires, conductors, LEDs, and movement cores.
Color: Mixed
Battery Required: No
Packing: 240 pcs
Box Specification: 360*360*415 mm

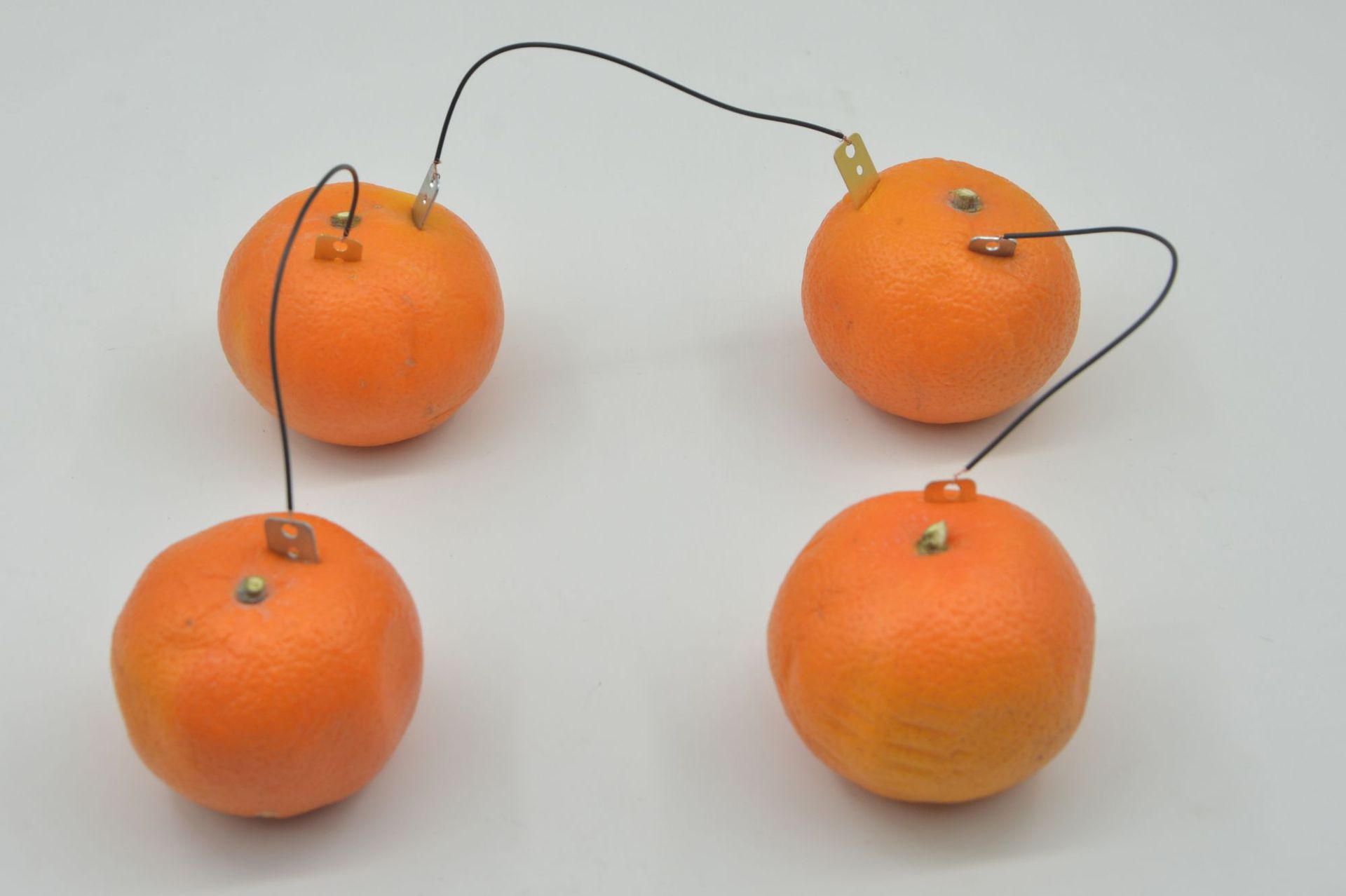
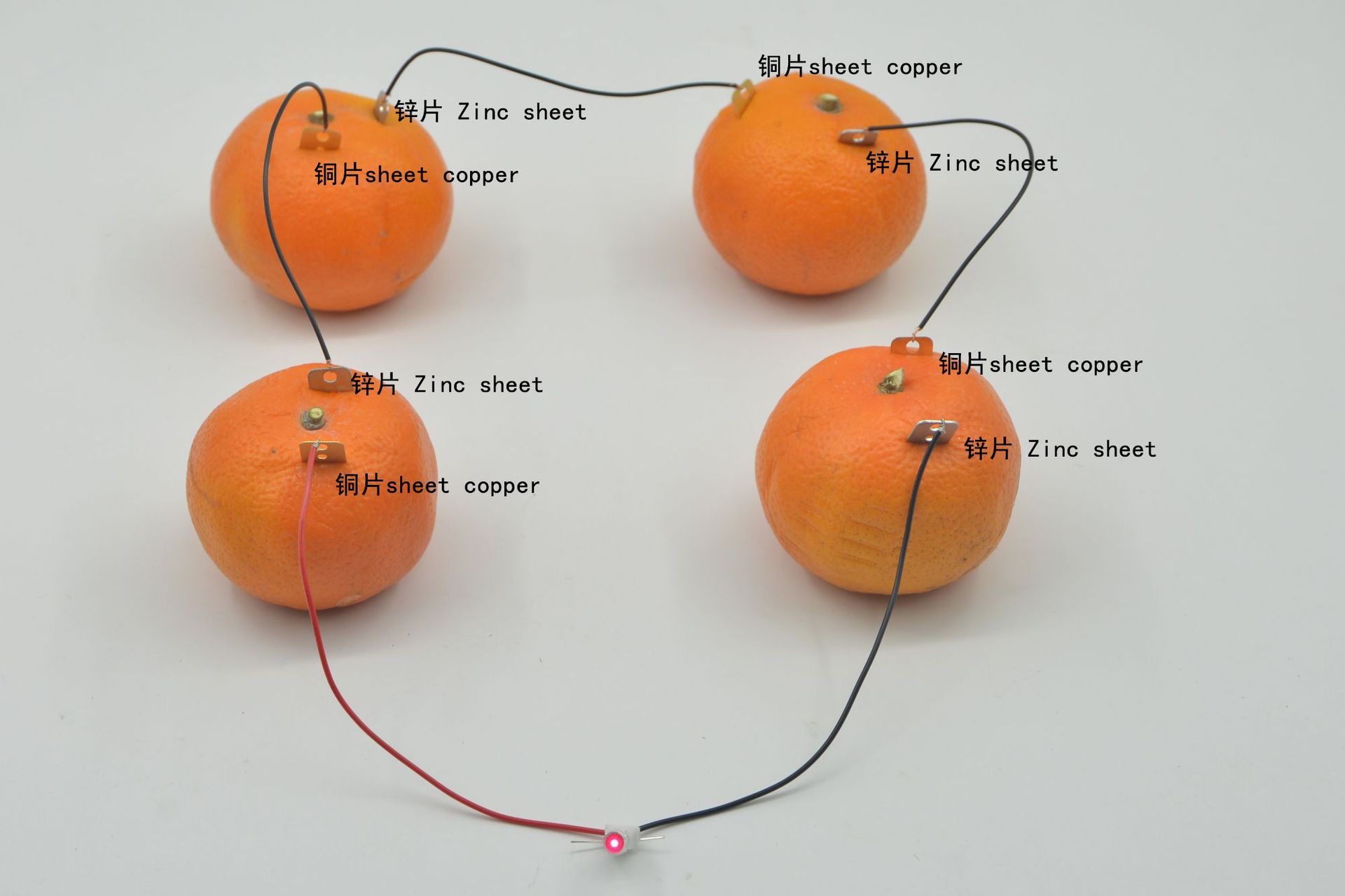
Electrode and Electrolyte
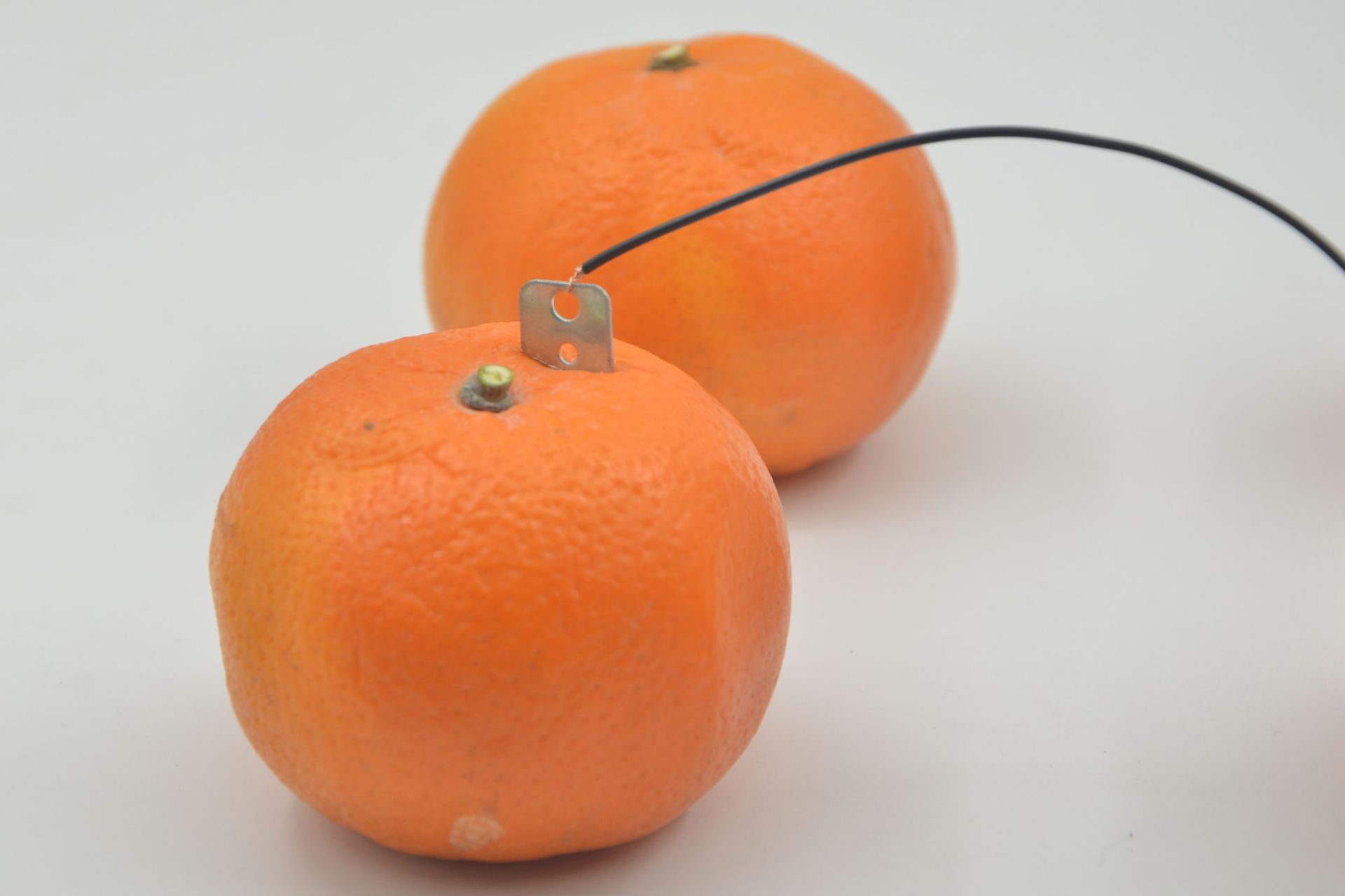
• Electrodes: In a fruit-based electricity generation device, two different metal plates are often inserted as electrodes, such as zinc plates and copper plates. The atomic structures of different metals are different, and they have different abilities to lose or gain electrons in an electrolyte solution.
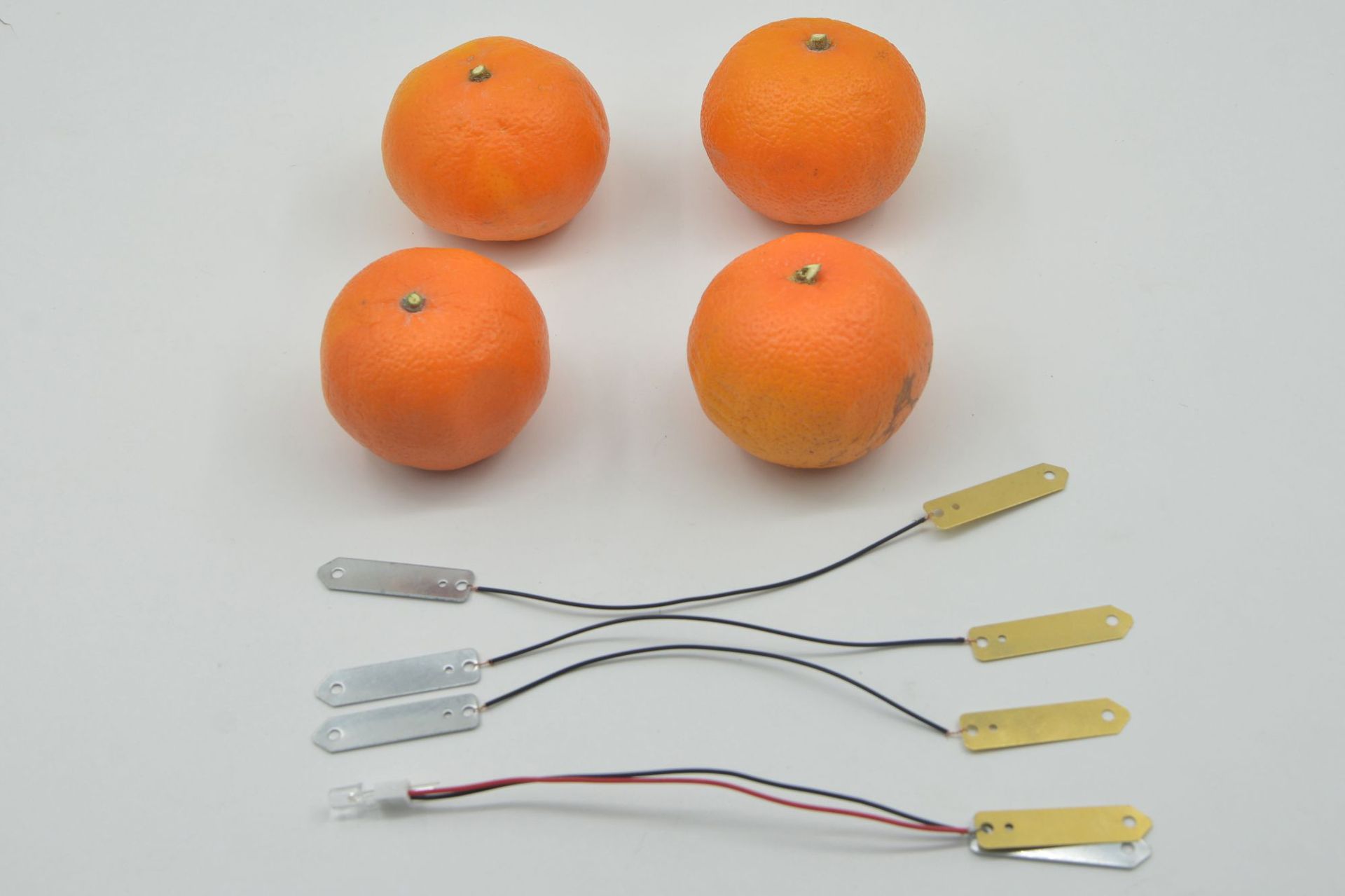
Electrolyte: The interior of fruits is rich in various electrolytes, such as organic acids like citric acid and malic acid, as well as minerals like potassium and sodium. These substances dissolve in the fruit's water to form an electrolyte solution.
Oxidation-Reduction Reaction
Zinc sheet reaction: In the electrolyte, zinc has a stronger metal activity, easily loses electrons to undergo oxidation reaction, turning into zinc ions entering the fruit electrolyte, the electrode reaction is: Zn - 2e^- = Zn^2+.
• Copper plate reaction: Some oxidizing substances in fruits gain electrons to occur reduction reactions on the surface of the copper plate. For example, under acidic conditions, hydrogen ions gain electrons to form hydrogen gas, and the electrode reaction formula is: 2H^{+}+2e^{-}=H_{2}\uparrow.
Electrons and Ions Move
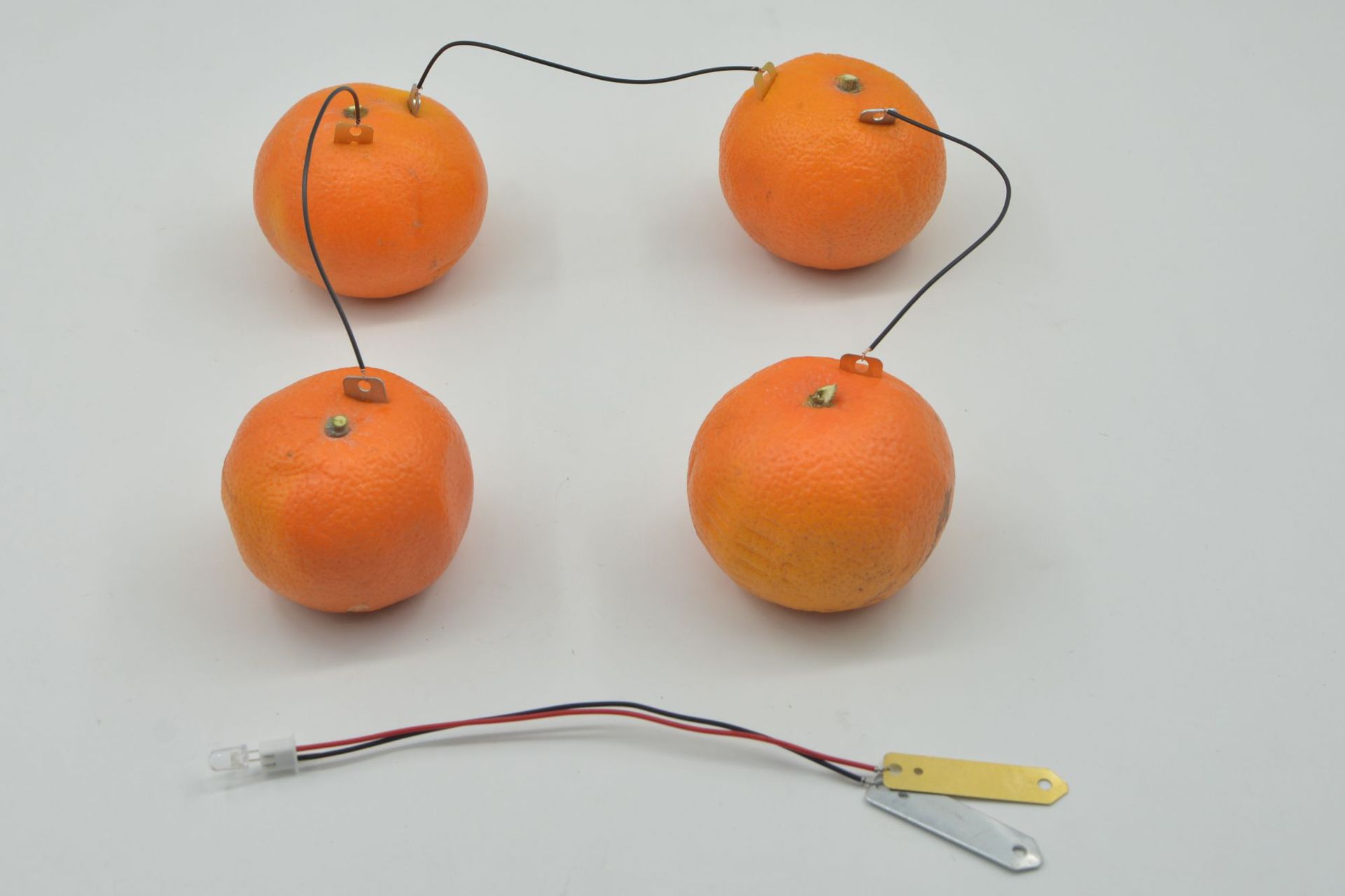
Electron movement: The electrons lost by the zinc piece flow through the external wire to the copper piece, thus forming an electron flow from the zinc piece to the copper piece, which is what we call electricity. This can make small electrical appliances such as light bulbs in the circuit glow or work.
Ion Movement: In the electrolyte within the fruit, to maintain charge balance, ions will move in a directed manner. Near the zinc plate, due to the increased number of zinc ions, the plate carries a positive charge, attracting the fruit's anions to move towards the zinc plate; near the copper plate, due to the hydrogen ions gaining electrons to form hydrogen, the positive charge decreases, attracting cations to move towards the copper plate.
Different fruits have different types and concentrations of electrolytes, which affect their power generation efficiency. Fruits with stronger acids, such as lemon juice, have higher concentrations of hydrogen ions, resulting in relatively higher power generation efficiency.
Step-by-step instructions: as follows.





Update time:
TOP