Search history
Clear allSearch by image
XDrag and drop an image here or upload an image
Max 5MB per image
UploadSign In | Join
Search history
Clear allSearch by image
XDrag and drop an image here or upload an image
Max 5MB per image
UploadSign In | Join
X Email Mobile
YiWu Medco Health Care Co.Ltd 10yr.
Contacts:Chen Yaling Chat
Mobile:86-18072445230
E-mail:sales@ywmedco.com.cn
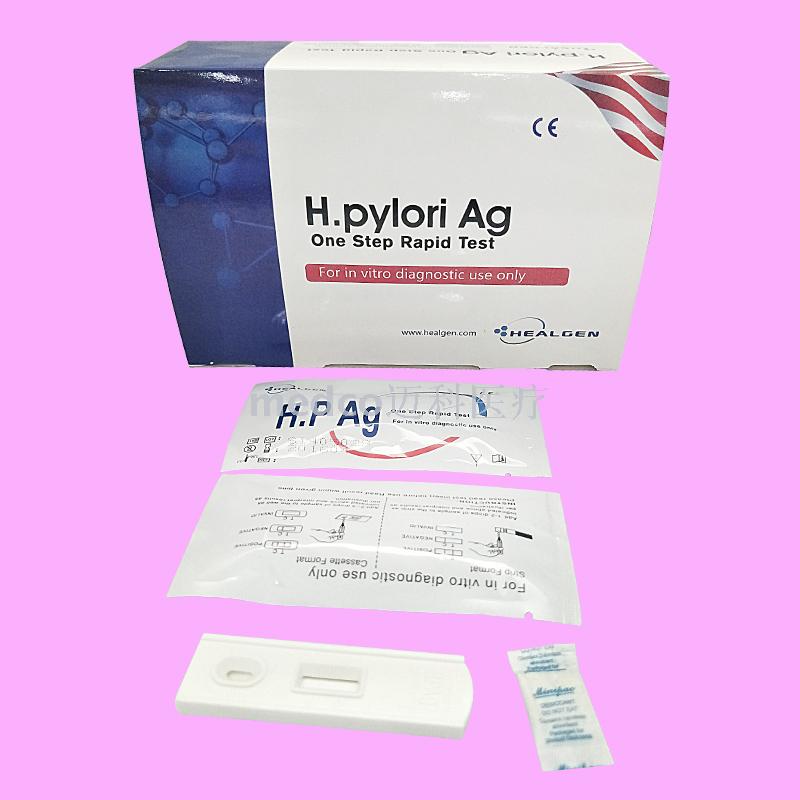
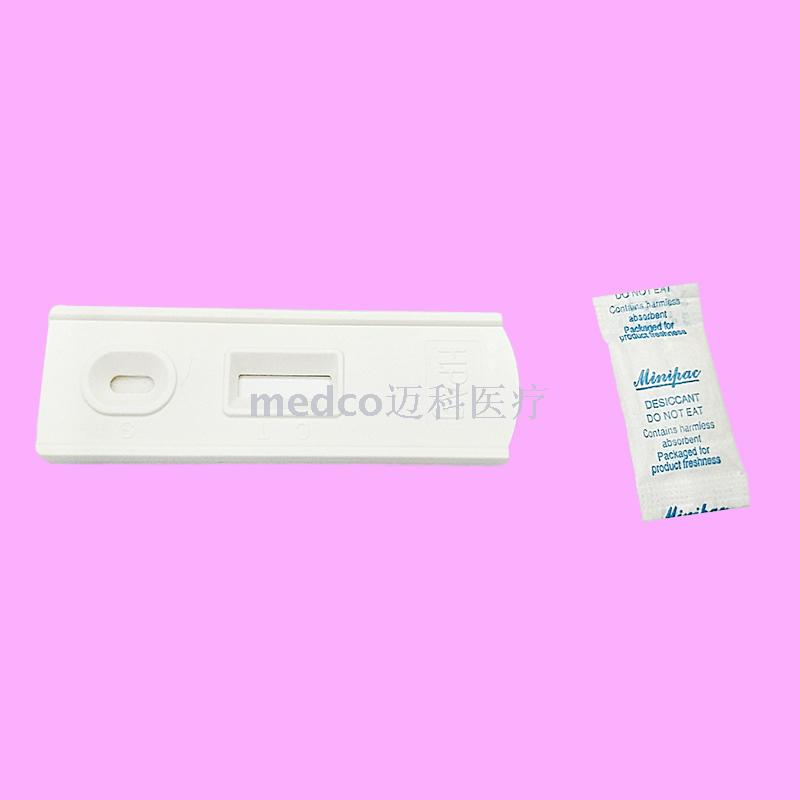

This product is a serum/plasma detection method that does not require any equipment. It USES the principle of immunochromatography to qualitatively detect the specific antibodies of helicobacter pylori in human serum and plasma. Auxiliary diagnosis of gastric disease symptoms of the case whether infected with helicobacter pylori, only for in vitro diagnosis.
[principle of measurement]
H. pyloriThe detection kit USES highly specific antibody antigen reaction and immunochromatography to qualitatively detect whether anti-helicobacter pylori antibodies are contained in serum/plasma. The test paper contains mouse anti-human antibodies prefixed in the membrane test area (T) and corresponding antibodies in the quality control area (C). During the test, the serum/plasma sample is dripped into the sample end of the test paper or into the sample hole (S) of the test paper. The serum/plasma sample is combined with the pre-coated latex particlesH. pyloriAntigen reaction. The mixture is then superimposed under the capillary effect. If it is positive, the emulsion will first bind to the anti-helicobacter pylori antibody in the specimen during the chromatography process, and then the conjugate will be fixed on the membrane to bind to the anti-human antibody of mice, and a red band will appear in the test area (T). If negative, there will be no red strip in the test area (T). A red band appears in the quality control area (C) regardless of whether antibodies against helicobacter pylori are present in the blood sample. The red strip shown in the quality control area (C) is the standard to determine whether there are enough samples and whether the chromatography process is normal, and also serves as the internal control standard of the reagent.
[equipment required for testing]
l The timer
l Disposable test tube
l Centrifuge (plasma only)
[specimen collection]
Serum or plasma is separated as soon as possible during specimen collection to avoid hemolysis. Test should be done with fresh specimens as far as possible. Specimens without inspection in time, can be in 2 ℃ 8 ℃ refrigerated for three days. Long-term preservation should be frozen in - 20 ℃, avoid repeated freezing and thawing
[operation steps]
Must first complete read the instructions before test, the test paper before use and blood samples back to room temperature (18 ℃ to 30 ℃).
1. Remove the test paper from the original aluminum foil bag and use it as soon as possible within 1 hour.
2. Test strip, insert the test paper into the sample, do not exceed the MAX mark line, or place the test paper on a clean and flat table surface for no less than 10 seconds, use a plastic straw to vertically add 3 drops (about 100ml) of serum/plasma without air bubbles into the sample hole (S) of the kit.
3. Wait for the magenta strip to appear and the test results should be read in 15 minutes. After 20 minutes, the verdict is invalid.
[result determination]
Positive (+):Two red strips appear.One is in the test area (T) and the other is in the quality control area (C). The positive results showed that the specimen contained antibodies against helicobacter pylori.
Negative (-):Only a red strip appears in the quality control area (C), no purplish red band appears in the test area (T). The negative results showed that antibodies against helicobacter pylori could not be detected in the samples.
invalid:No red strip appears in the quality control area (C).Indicates incorrect operation or deterioration of the kit. In this case, read the instructions carefully again and retest with a new kit. If the problem persists, stop using the lot number immediately and contact the local supplier.
Note:The fuchsia strips in the test area (T) show different shades of color
Color. However, in the specified observation time, regardless of the color band color depth, namely
So that only very weak bands should also be judged as a positive result.
Sensitivity, specificity and accuracy
useH. pyloriThe detection kit detects clinically collected serum and plasma samples from symptomatic and asymptomatic individuals undergoing endoscopic examination, with biological methods (culture and/or histology) as the reference test. The relative sensitivity, specificity and accuracy of the samples were 94.2%, 76.8% and 86.2%, respectively.
[cross reaction]
Campylobacter jejuni, campylobacter foetus, campylobacter colon and e. coli all contain a certain amount of helicobacter pylori antibodies. No cross-reaction was found after the detection of these bacteria, indicating ACONH. pyloriThe detection kit is highly specific for the detection of human helicobacter pylori antibody.
[usage restrictions]
1.H. pyloriThe test kit is only used for in vitro diagnosis and is only used to detect antibodies in serum or plasma samples.
2.H. pyloriThe detection kit only proved the presence of helicobacter pylori antibodies in the specimens, which could not be used as the sole criterion for determining helicobacter pylori infection.
3.H. pyloriThe diagnostic result of the detection kit is the auxiliary diagnosis of clinical detection.
4. The test results are negative and the clinical symptoms still exist. Further clinical tests should be conducted. Negative test results still cannot exclude helicobacter pylori infection.
Update time:
TOP