Search history
Clear allSearch by image
XDrag and drop an image here or upload an image
Max 5MB per image
UploadSign In | Join
Search history
Clear allSearch by image
XDrag and drop an image here or upload an image
Max 5MB per image
UploadSign In | Join
X Email Mobile
YiWu Medco Health Care Co.Ltd 10yr.
Contacts:Chen Yaling Chat
Mobile:86-18072445230
E-mail:sales@ywmedco.com.cn
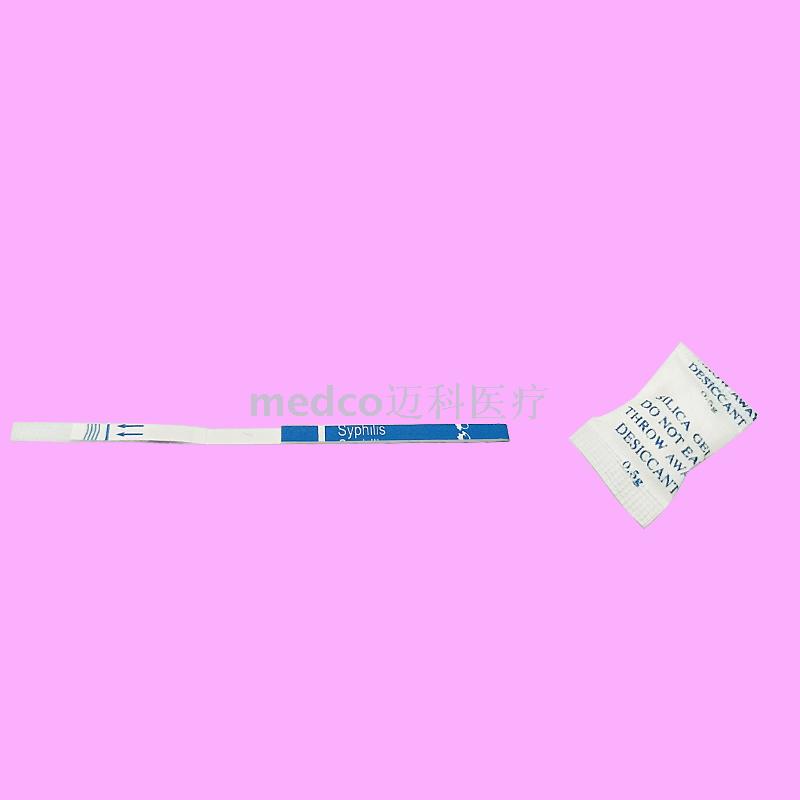
This product is a whole blood / serum / plasma detection reagent that does not need any instruments and equipment.
It uses the principle of immunochromatographic analysis to quickly detect whether the whole blood / serum / plasma contains syphilis antibodies, thus helping the clinical judgment of whether the human body is infected by Treponema pallidum.

|
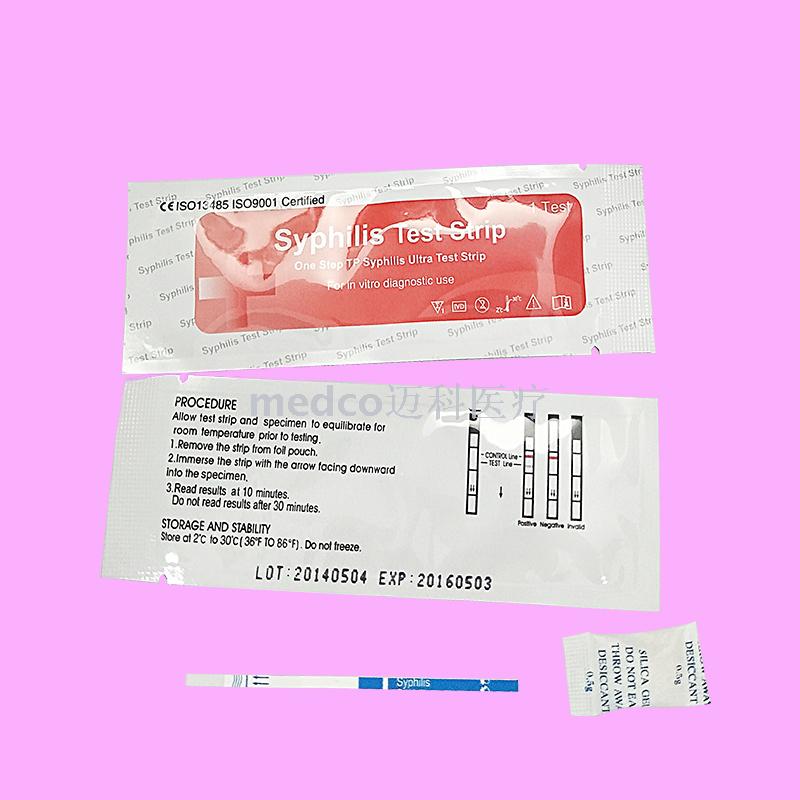
This product is a kind of whole blood/serum/plasma detection reagent that does not need any equipment. It USES the principle of immunochromatography analysis to quickly detect whether the whole blood/serum/plasma contains syphilis antibodies, so as to assist clinical judgment of whether the human body is infected by treponema pallidum. [principle of measurement]The syphilis kit USES highly specific antibody antigen reaction and immunochromatography to qualitatively detect whether syphilis antibody is contained in the whole blood/serum/plasma. The kit contains the recombinant specific syphilis spiral antibody antigen which is fixed on the membrane test area (T) and the corresponding antibody in the quality control area (C). During the test, the whole blood/serum/plasma samples were dripped into the kit plus sample orifice (S), and the antibody of treponema pallidum in the samples reacted with the labeled binding compound precoated on the membrane. The mixture was then superimposed with capillarity to react with the recombinant treponema pallidum antigen fixed to the membrane at the test site (T). If the specimen contains antibodies to treponema pallidum, a red band will appear in the test area (T) indicating a positive result. If there is no red band in the test area (T), the blood sample does not contain antibodies against treponema pallidum, indicating a negative result. Regardless of the presence of anti-treponema pallidum antibodies in the study specimen, the mixture will continue to be analyzed to the upper layer to the quality control area (C), where the corresponding antibody reacts with the labeled binding material in a red band. The red strip shown in the quality control area (C) is the standard to determine whether the chromatography process is normal, and also serves as the internal control standard of the kit. [specimen collection]Serum or plasma is separated as soon as possible during specimen collection to avoid hemolysis. Fresh specimens should be used when testing. Specimens without inspection in time, can be in 2 ℃ 8 ℃ refrigerated for three days, whole blood for two days. Long-term preservation should be frozen in - 20 ℃, avoid repeated freezing and thawing, whole blood specimens should not be frozen. [operation steps] Must complete the reading before test instruction for use, before using the test kit and whole blood/serum/plasma samples back to room temperature (20 ℃ to 30 ℃). 1. Remove the kit from the original packaging aluminum foil bag and use it as soon as possible within one hour. 2. Add sample and strip: place the reagent on a clean and flat surface, add two drops of samples vertically and put them in the sampling area, add one drop of buffer solution, and start timing. 3. Wait for the emergence of the red strip, and the test results shall be interpreted within 10-30 minutes. After 30 minutes, the decision is invalid [result determination] The positive (+) : two red strips appear. One is in the test area (T) and the other is in the quality control area (C). The positive results showed that the specimen contained antibodies against syphilis. Negative (--) : only the quality control area (C)), a red band appears, no purplish red band appears in the test area (T). Negative results showed that syphilis antibody could not be detected in the specimen. Invalid: quality control area (C)) no red bands appear, indicating incorrect operation or deterioration of the reagent strip. In this case, read the instructions carefully again and retest with a new kit. If the problem persists, stop using the lot number immediately and contact the local supplier. Pay attention to: due to the different titers of syphilis antibodies in the samples, the red bands in the test area (T) will show different shades of color. However, the test results of this reagent bar cannot be used as the basis for determining the titer of antibody in the sample. |
\"Please read the product manual carefully or purchase and use it under the guidance of the medical staff\".
Update time:
TOP